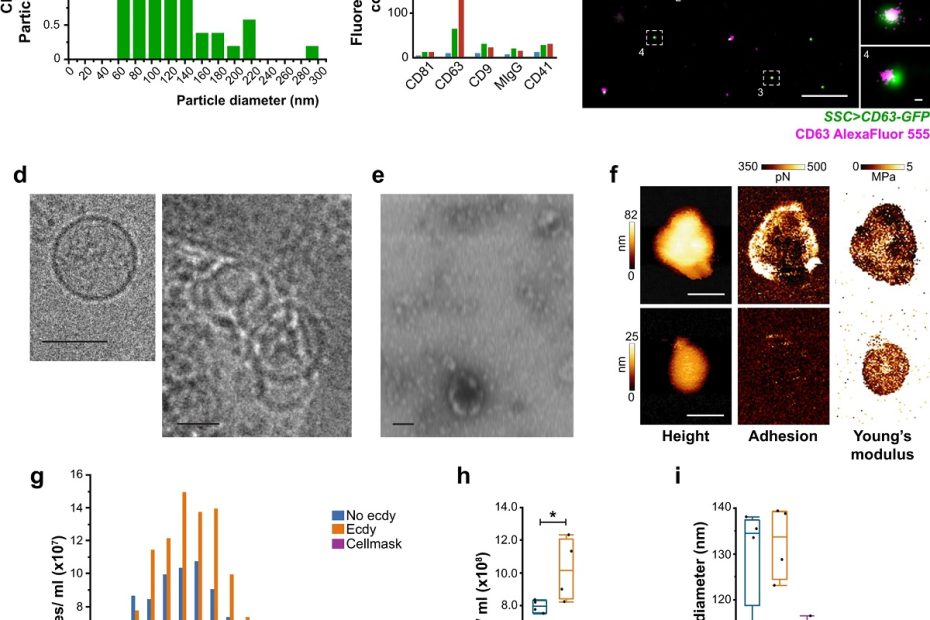
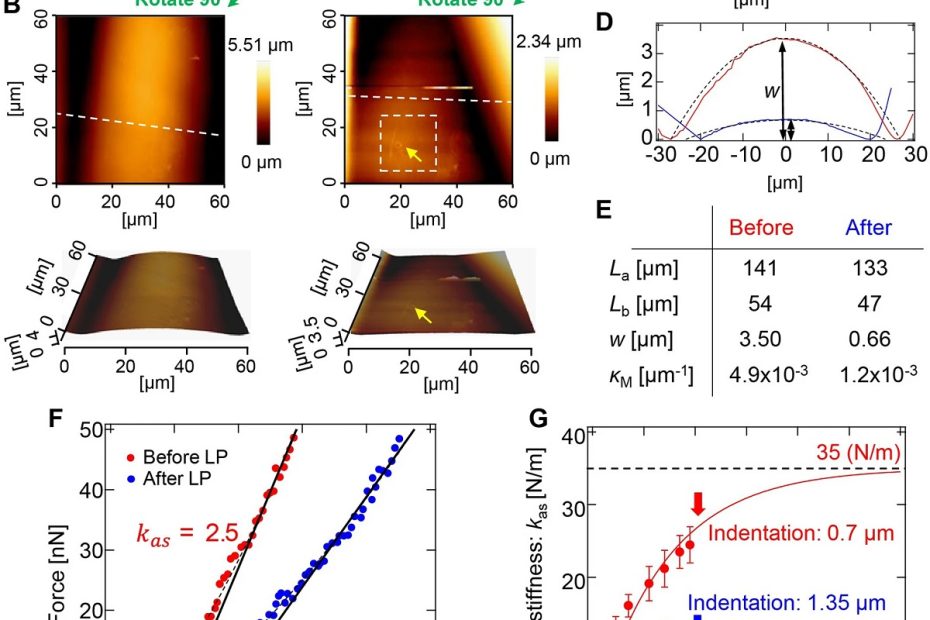
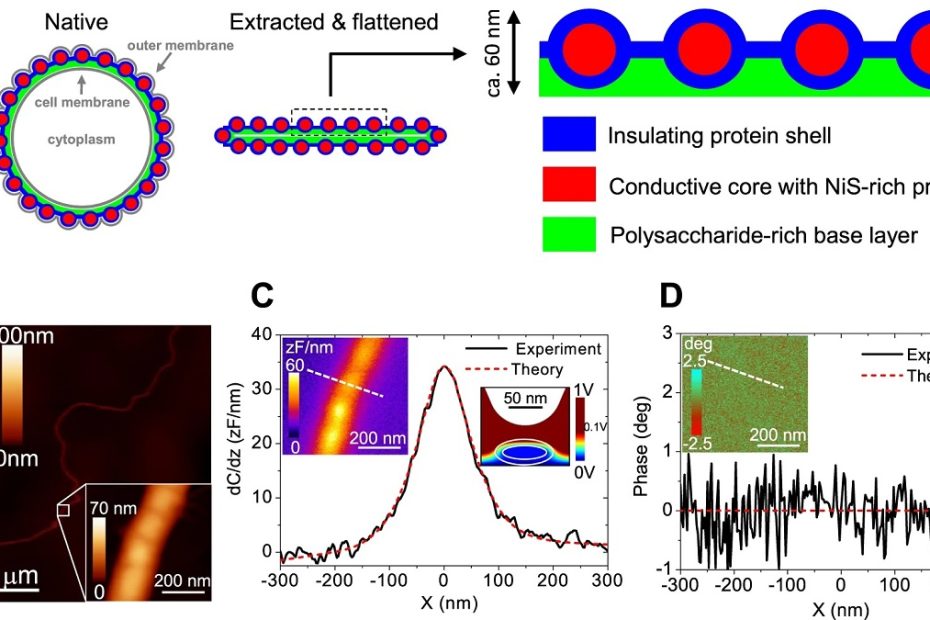
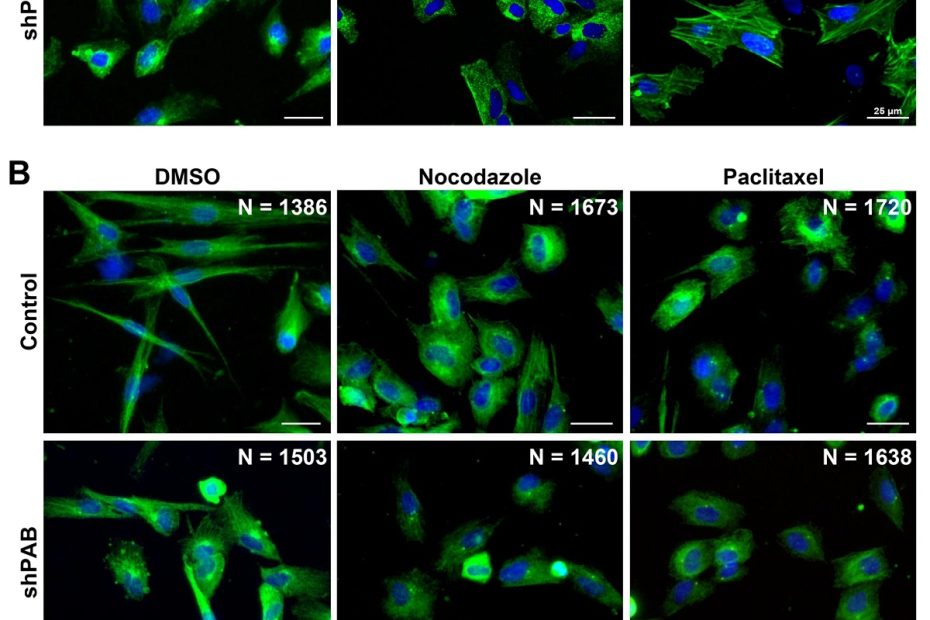
Mitochondrial-derived vesicles retain membrane potential and contain a functional ATP synthase
Vesicular transport is a means of communication. * While cells can communicate with each other via secretion of extracellular vesicles, less is known regarding organelle‐to… Read More »Mitochondrial-derived vesicles retain membrane potential and contain a functional ATP synthase