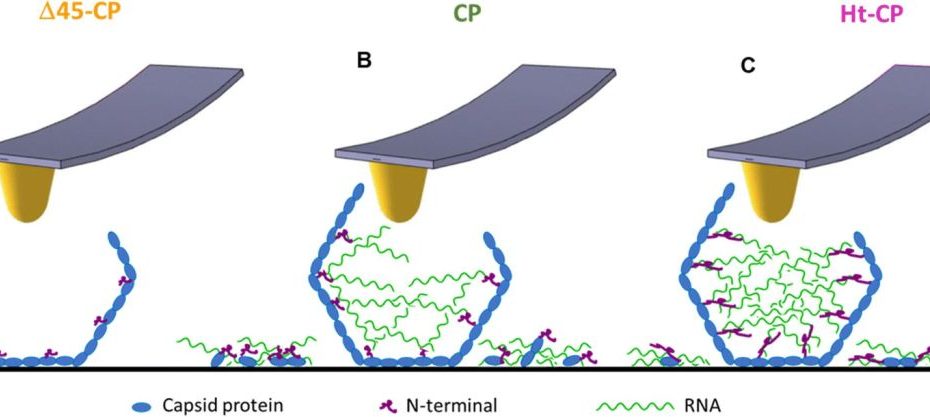
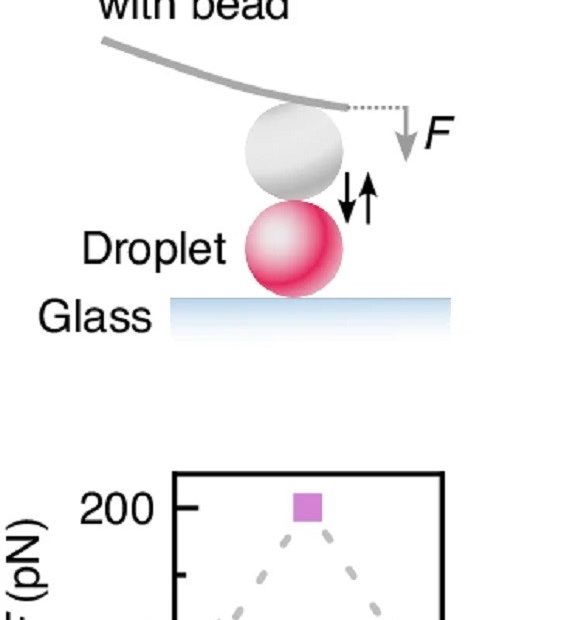
Mechanical disassembly of human picobirnavirus like particles indicates that cargo retention is tuned by the RNA-coat protein interaction
The idea of using virus-like particles as nanocarriers for heterologous cargo transport and delivery requires controlling the stability of the container–cargo system.* In particular, the… Read More »Mechanical disassembly of human picobirnavirus like particles indicates that cargo retention is tuned by the RNA-coat protein interaction
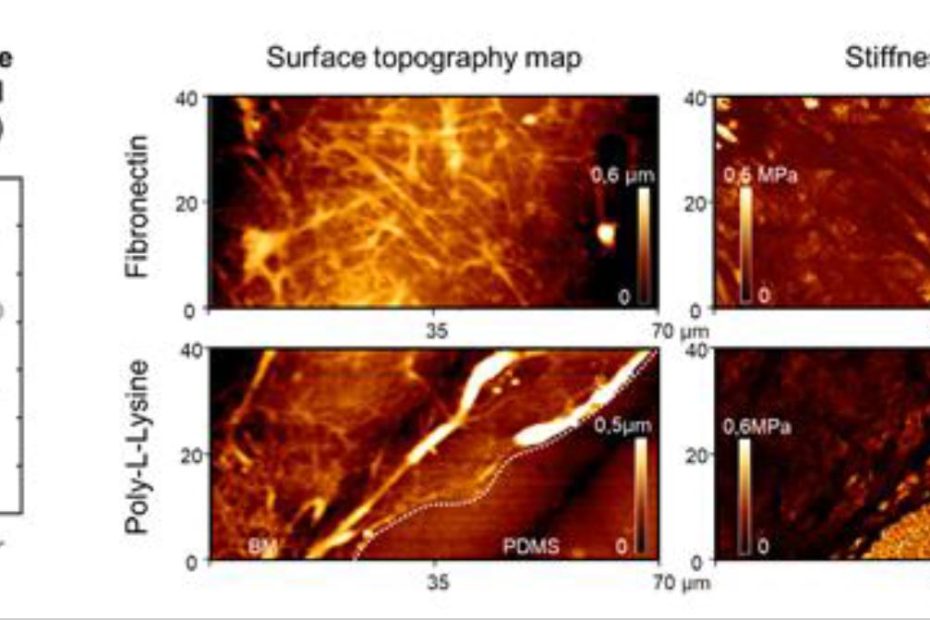
![Figure 5 from Catharina Husteden et al. 2023 “Lipoplex-Functionalized Thin-Film Surface Coating Based on Extracellular Matrix Components as Local Gene Delivery System to Control Osteogenic Stem Cell Differentiation”: A–D) Topography images [Cs/Col]4Cs (A), [Cs/Col]4Cs/LPX (B), [Cs/Col]4Cs/LPX/Cs (C), and [Cs/Col]4Cs/LPX/Cs/Col determined by AFM [Scale bar 1 µm] (D). E) Distribution curves of Young's modulus (E0) with a force map of a defined area (see also Figure S4, Supporting Information of the cited article). NANOSENSORSTM uniqprobe triangular qp-BioT AFM probes were used for the quantitative imaging to investigate the surface roughness and topography (in a standard liquid cell of a commercially available atomic force microscope) and force mapping. The uniqprobe qp-BioT AFM probe types offer an alternative to silicon nitride probes, with the advantage of taller AFM tips with smaller opening angles and reduced thermal drift.](https://d218f3btfcac6d.cloudfront.net/wp-content/uploads/2024/05/16205027/Fig-5-C-Husteden-et-al-2023-Lipoplex-Functionalized-Thin-Film-Surface-Coating-Based-on-Extracellular-Matrix-Components-NANOSENSORS-qp-BioT-AFM-probe-blog-930x620.jpg)


