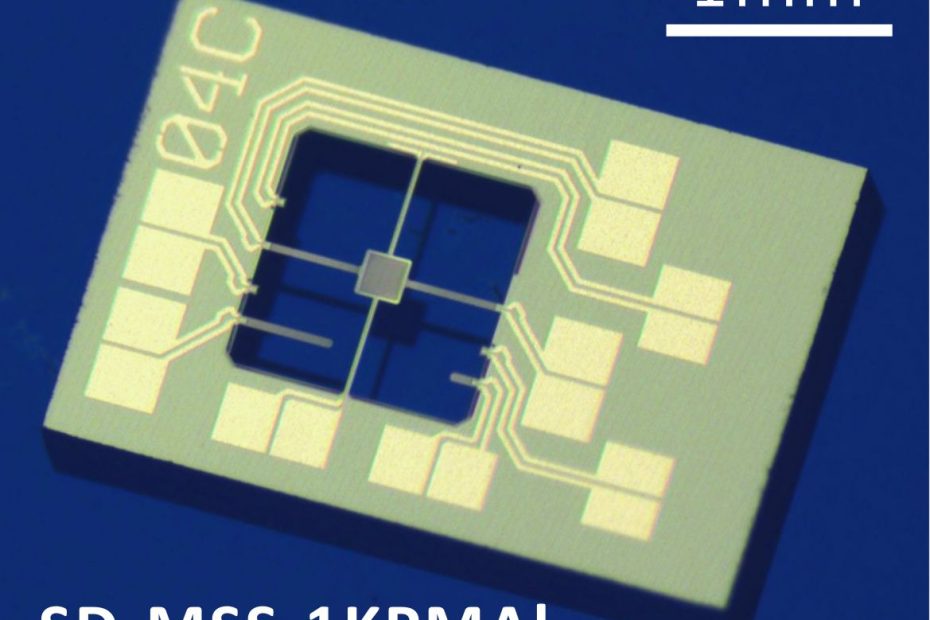
Two New MSS Sensors for Torque Magnetometry added to Special Developments List
NANOSENSORS™ has completed the development of two additional Membrane-type Surface-stress Sensors (MSS) ( SD-MSS-1KPMAl and SD-MSS-1KPMAu ) dedicated for torque magnetometry. Torque magnetometry is a… Read More »Two New MSS Sensors for Torque Magnetometry added to Special Developments List