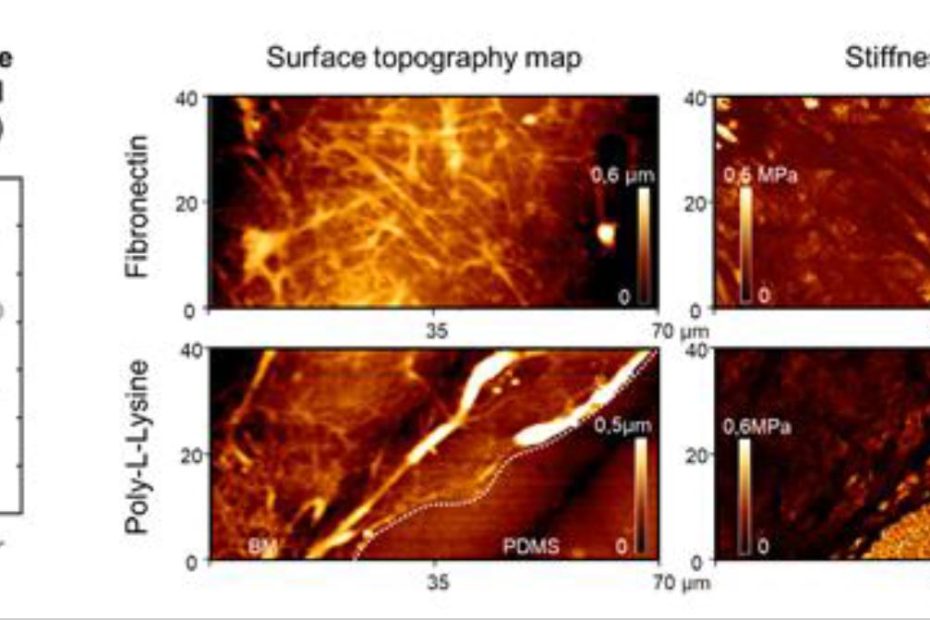
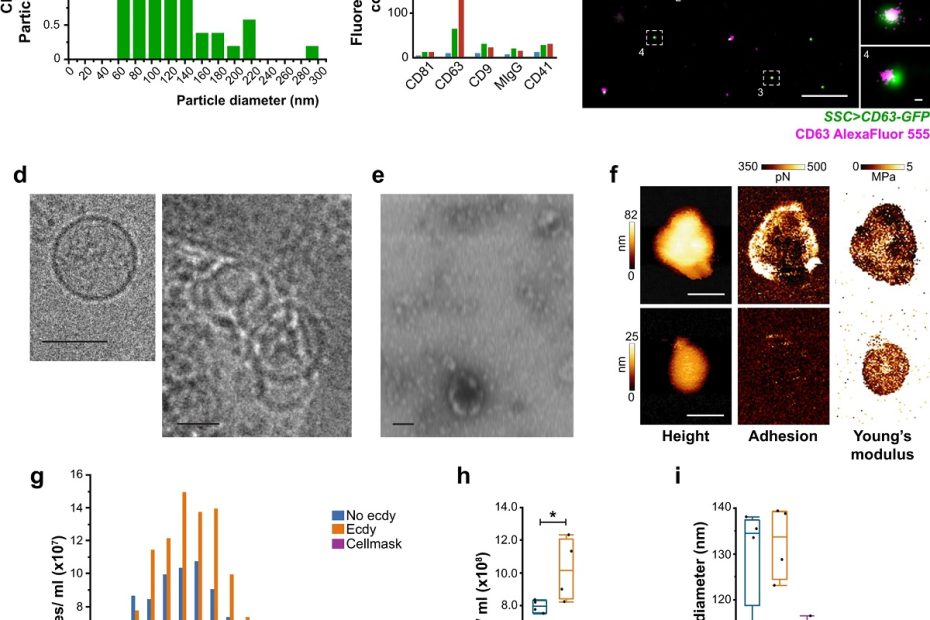
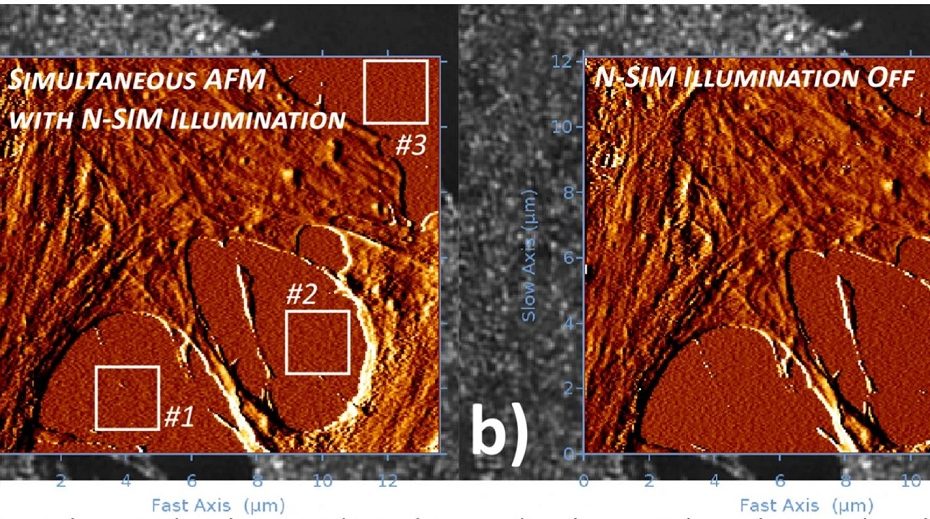
Distinct Contact Guidance Mechanisms in Single Endothelial Cells and in Monolayers
In many tissues, cell shape and orientation are controlled by a combination of internal and external biophysical cues. * Cell shape is closely controlled in… Read More »Distinct Contact Guidance Mechanisms in Single Endothelial Cells and in Monolayers