Correlating data from different microscopy techniques holds the potential to discover new facets of signalling events in cellular biology.*
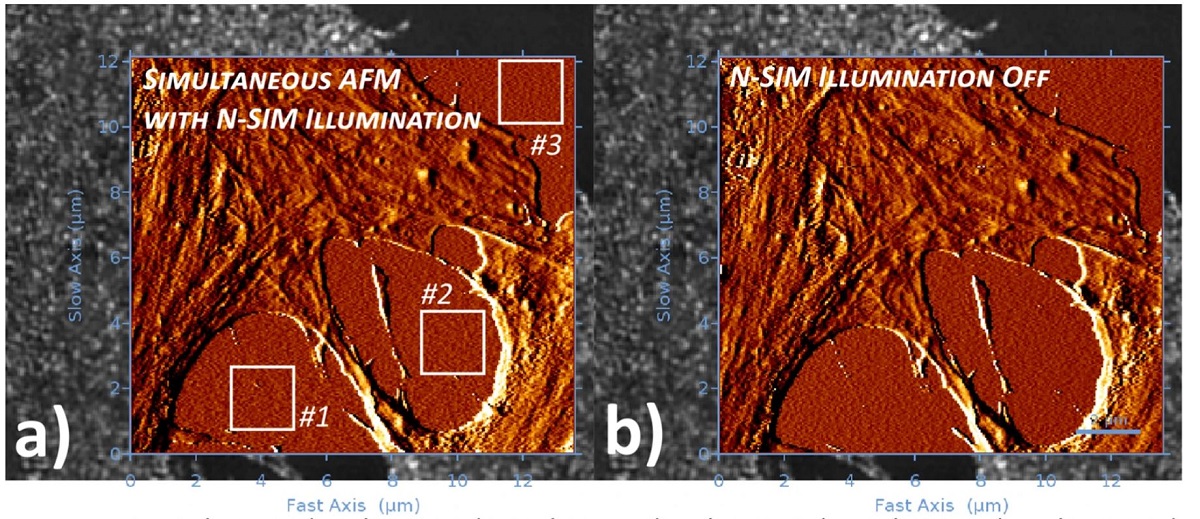
In the article “Simultaneous co-localized super-resolution fluorescence microscopy and atomic force microscopy: combined SIM and AFM platform for the life sciences” Ana I. Gómez-Varela, Dimitar R. Stamov, Adelaide Miranda, Rosana Alves, Cláudia Barata-Antunes, Daphné Dambournet, David G. Drubin, Sandra Paiva and Pieter A. A. De Beule report for the first time a hardware set-up capable of achieving simultaneous co-localized imaging of spatially correlated far-field super-resolution fluorescence microscopy and atomic force microscopy, a feat only obtained until now by fluorescence microscopy set-ups with spatial resolution restricted by the Abbe diffraction limit.*
The authors detail system integration and demonstrate system performance using sub-resolution fluorescent beads and applied to a test sample consisting of human bone osteosarcoma epithelial cells, with plasma membrane transporter 1 (MCT1) tagged with an enhanced green fluorescent protein (EGFP) at the N-terminal.*
The simultaneous operation of AFM and super-resolution fluorescence microscopy technique provides a powerful observational tool on the nanoscale, albeit data acquisition is typically obstructed by a series of integration problems. The authors of the above-mentioned article believe that the combination of SR-SIM with AFM presents one of the most promising schemes enabling simultaneous co-localized imaging, allowing the recording of nanomechanical data and cellular dynamics visualization at the same time.*
For measurements on cells in liquid NANOSENSORS™ uniqprobe qp-BioAC-CI AFM probes ( CB1 ) with a nominal resonance frequency of 90 kHz (in air), spring constant of 0.3 Nm−1, partial gold coating on the detector side, and quartz-like circular symmetric hyperbolic (double-concaved) tips with ROC of 30 nm were used. The corresponding AFM areas for the cell images were acquired with a Z-cantilever velocity of 250 μms−1 at a max Z-length of 1.5 μm, resulting in an acquisition time (based on the number of pixels) for Figs. 2, 3, 4 of ca. 13, 8 and 15 min respectively.*
*Ana I. Gómez-Varela, Dimitar R. Stamov, Adelaide Miranda, Rosana Alves, Cláudia Barata-Antunes, Daphné Dambournet, David G. Drubin, Sandra Paiva and Pieter A. A. De Beule
Simultaneous co-localized super-resolution fluorescence microscopy and atomic force microscopy: combined SIM and AFM platform for the life sciences
Nature Scientific Reports volume 10, Article number: 1122 (2020)
DOI: https://doi.org/10.1038/s41598-020-57885-z
Please follow this external link to read the full article https://rdcu.be/b4Iot
Open Access: The article “Simultaneous co-localized super-resolution fluorescence microscopy and atomic force microscopy: combined SIM and AFM platform for the life sciences” by Ana I. Gómez-Varela, Dimitar R. Stamov, Adelaide Miranda, Rosana Alves, Cláudia Barata-Antunes, Daphné Dambournet, David G. Drubin, Sandra Paiva and Pieter A. A. De Beule is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.