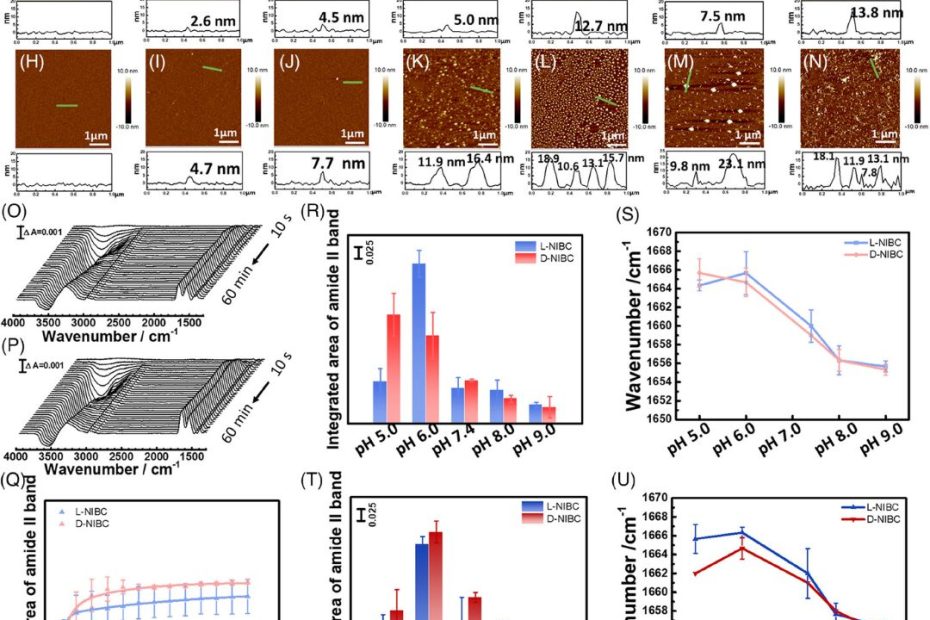
Revealing the regulation of water dipole potential to aggregation of amyloid-β at chiral interface by surface-enhanced infrared adsorption spectroscopy
Surface chirality plays an important role in determining the biological effect. Understanding how surface chirality regulates the behaviors of biological entities such as biomolecules and… Read More »Revealing the regulation of water dipole potential to aggregation of amyloid-β at chiral interface by surface-enhanced infrared adsorption spectroscopy