Yeast resistance to antifungal drugs is a major public health issue. Fungal adhesion onto the host mucosal surface is still a partially unknown phenomenon that is modulated by several actors among which fibronectin plays an important role. Targeting the yeast adhesion onto the mucosal surface could lead to potentially highly efficient treatments. *
In the article “Yeast Nanometric Scale Oscillations Highlights Fibronectin Induced Changes in C. Albicans” Anne-Céline Kohler, Leonardo Venturelli, Abhilash Kannan, Dominique Sanglard, Giovanni Dietler, Ronnie Willaert and Sandor Kasas, explore the effect of fibronectin on the nanomotion pattern of different Candida albicans strains by atomic force microscopy ( AFM )-based nanomotion detection and correlated the cellular oscillations to the yeast adhesion onto epithelial cells. *
The authors demonstrate the ability of nanomotion detection to monitor in real time and in a label-free manner cellular activity changes induced by interacting ligands. Activity changes induced by increasing glucose concentration were observed for Escherichia coli in a previous study. This technique opens novel avenues to detect cellular activation or inhibition induced by ligand–receptor interactions. *
The AFM cantilever oscillations were collected in real time using an by the authors in-house developed nanomotion detection device with NANOSENSORS™ tipless uniqprobe SD-qp-CONT-TL AFM probes from the NANOSENSORS Special Developments List. *
The SD-qp-CONT-TL AFM cantilevers with a nominal spring constant of 0.1 N/m and an average resonant peak in liquids of 8 kHz, were coated with 2 mg/mL of concanavalin A (Con A) for 30 min at room temperature. After removing the excess of Con A, the yeast cells were placed in contact with the AFM cantilever for 1 h at room temperature to allow them to attach to its surface. Poorly attached C. albicans cells were removed by washing gently with YPD medium. Finally, the C. albicans covered AFM cantilever was inserted into the analysis chamber containing 2 mL of filtered (0.2 µm syringe filter, YPD medium. The measurements were performed at room temperature in YPD medium and in YPD medium containing 25 µg/mL of fibronectin. Fibronectin was directly added inside the chip reservoir. For the experiments performed with antifungals, caspofungin was diluted in the YPD present in the analysis chamber to reach a final concentration of 100 µg/mL. *
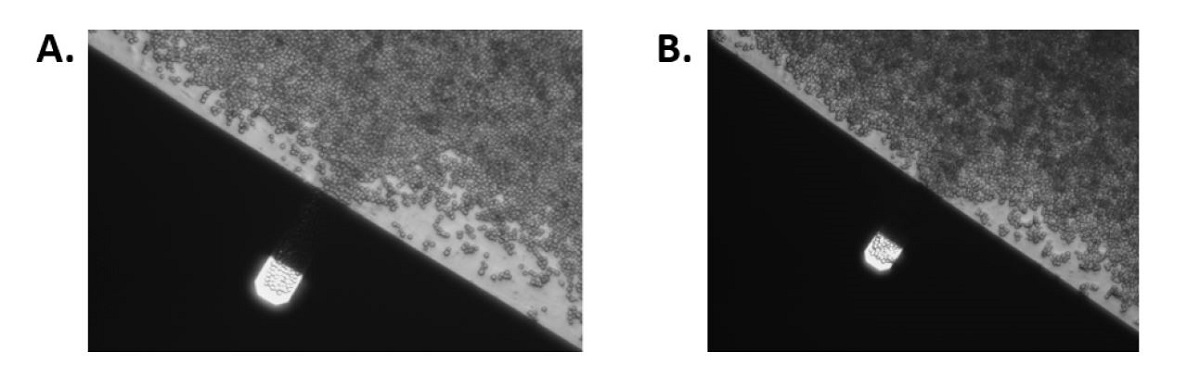
Density of yeast cells on cantilever at the start of the experiment (A) and at the end of the experiment (B). The size of the AFM cantilever is 40 by 130 μm.
*Anne-Céline Kohler, Leonardo Venturelli, Abhilash Kannan, Dominique Sanglard, Giovanni Dietler, Ronnie Willaert and Sandor Kasas
Yeast Nanometric Scale Oscillations Highlights Fibronectin Induced Changes in C. Albicans
Fermentation 2020, 6(1), 28
DOI: https://doi.org/10.3390/fermentation6010028
Please follow this external link to read the full article: https://www.mdpi.com/2311-5637/6/1/28/htm#B5-fermentation-06-00028
Open Access The article “Yeast Nanometric Scale Oscillations Highlights Fibronectin Induced Changes in C. Albicans” by Anne-Céline Kohler, Leonardo Venturelli, Abhilash Kannan, Dominique Sanglard, Giovanni Dietler, Ronnie Willaert and Sandor Kasas is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.