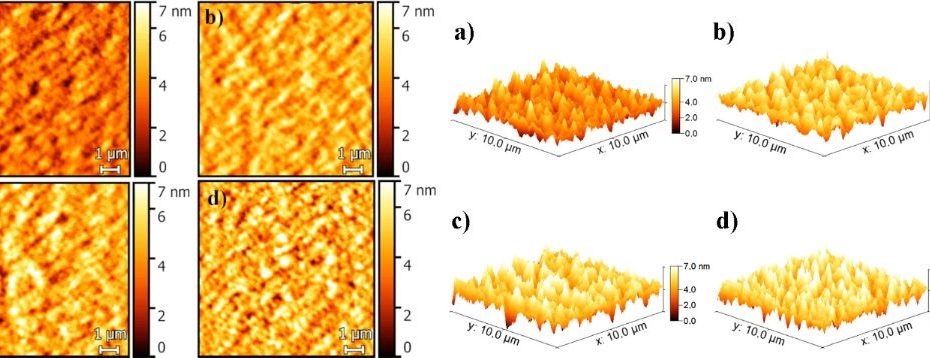
Nontoxic pyrite iron sulfide nanocrystals as second electron acceptor in PTB7:PC71BM-based organic photovoltaic cells
Iron disulfide ( FeS2 ) is a natural earth-abundant and nontoxic material with possible applications in lithium batteries, transistors or photovoltaic (PV) devices. According to… Read More »Nontoxic pyrite iron sulfide nanocrystals as second electron acceptor in PTB7:PC71BM-based organic photovoltaic cells