Mechanical forces have emerged as coordinating signals for most cell functions. Yet, because forces are invisible, mapping tensile stress patterns in tissues remains a major challenge in all kingdoms.*
In their research paper “A tension-adhesion feedback loop in plant epidermis” Stéphane Verger, Yuchen Long, Arezki Boudaoud and Olivier Hamant take advantage of the adhesion defects in the Arabidopsis mutant quasimodo1 (qua1) to deduce stress patterns in tissues.*
Using suboptimal water potential conditions, the authors revealed the relative contributions of shape- and growth-derived stress in prescribing maximal tension directions in aerial tissues. Consistently, the tension patterns deduced from the gaping patterns in qua1 matched the pattern of cortical microtubules, which are thought to align with maximal tension, in wild-type organs. Conversely, loss of epidermis continuity in the qua1mutant hampered supracellular microtubule alignments, revealing that coordination through tensile stress requires cell-cell adhesion.*
Based on the results achieved with plants presented in this research paper, the analysis of basement membrane continuity, and its disruption, may very well help understand how consistent supracellular epidermal patterns relate to mechanical stress, in parallel to the well-established role of cadherin and stress in cell-cell adhesion and epidermal functions.*
NANOSENSORS™ special development Sphere AFM probes ( SD-Sphere-NCH-S ) with 400 nm tip radius and 42 N/m spring constant were used.
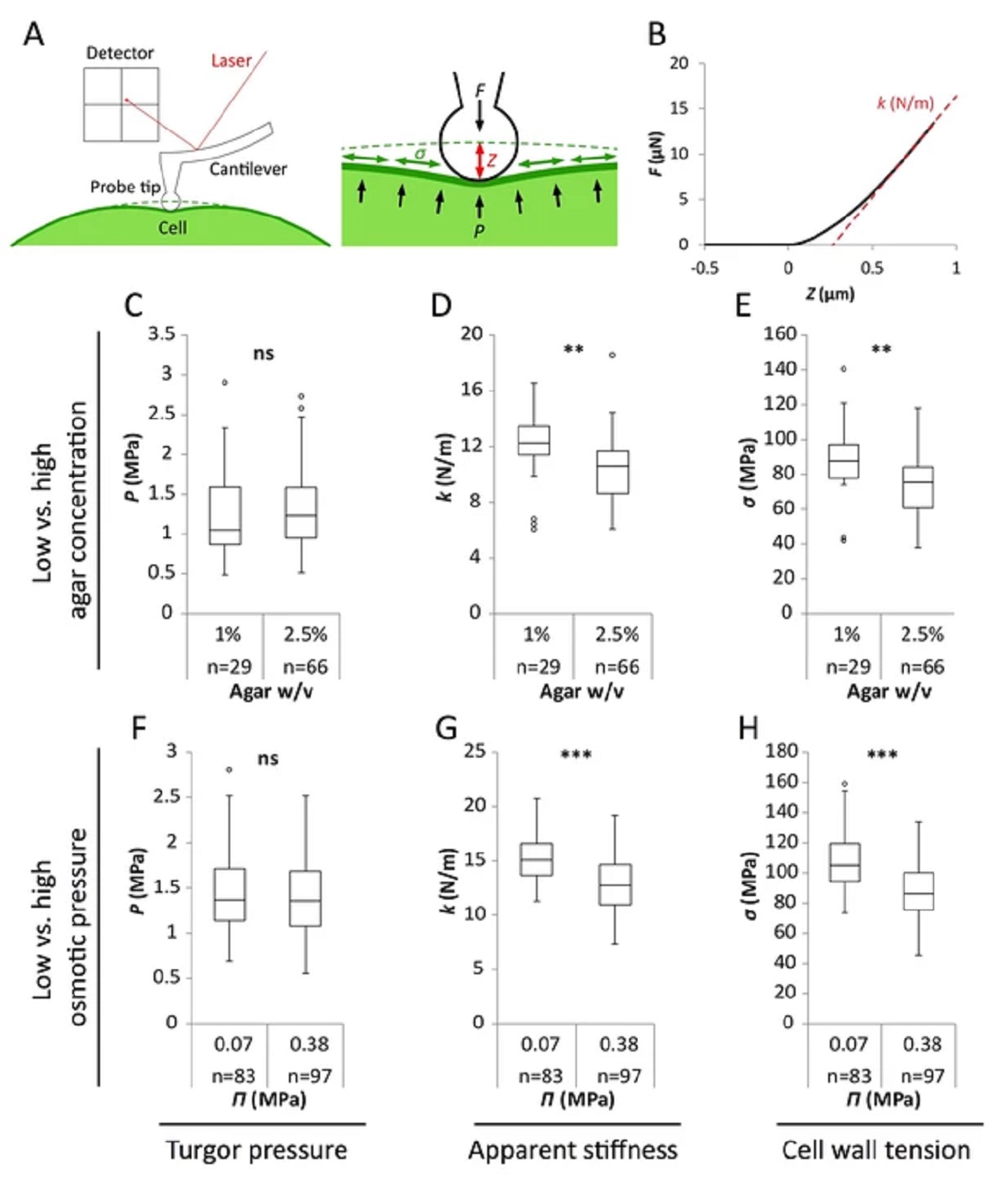
Figure 2 from Stéphane Verger et al. “A tension-adhesion feedback loop in plant epidermis”:
Reduced water potential in growth medium causes decrease in pavement cell stiffness and cell wall tension, not turgor pressure.
(A) Schematic representation of AFM nano-indentation principle of measurements. F, indentation force; Z, indentation depth; P, turgor pressure; σ, cell wall tension. (B) Example of a typical AFM force curve (black line) and linear fit at deep indentation (red dotted line, 75 ~ 99% of maximum force) which depicts apparent stiffness k. (C–H) Box plots of the turgor pressure P (C,F), apparent stiffness k (D,G) and cell wall tension σ (E,H) of cotyledon pavement cells grown on medium with differential agar concentration (1% and 2.5% w/v) (C–E) or osmotic pressure Π (0.07 and 0.38 MPa) (F–H). Circles indicate Tukey’s outliers. Student’s t-test, ** indicates p-value<0.01; ***, p-value<0.001; ns, not significant; n, number of measured cells.
*Stéphane Verger, Yuchen Long, Arezki Boudaoud, Olivier Hamant
A tension-adhesion feedback loop in plant epidermis
eLife 2018;7:e34460
DOI: https://doi.org/10.7554/eLife.34460
Please follow this external link to read the full article: https://cdn.elifesciences.org/articles/34460/elife-34460-v3.pdf
Open Access: The article “A tension-adhesion feedback loop in plant epidermis” by Stéphane Verger, Yuchen Long, Arezki Boudaoud and Olivier Hamant is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/