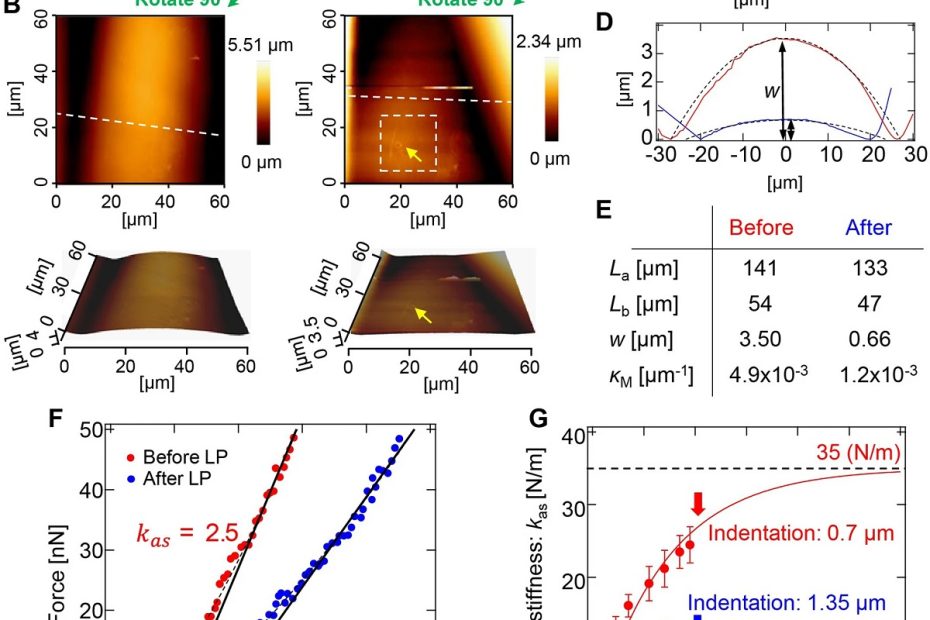
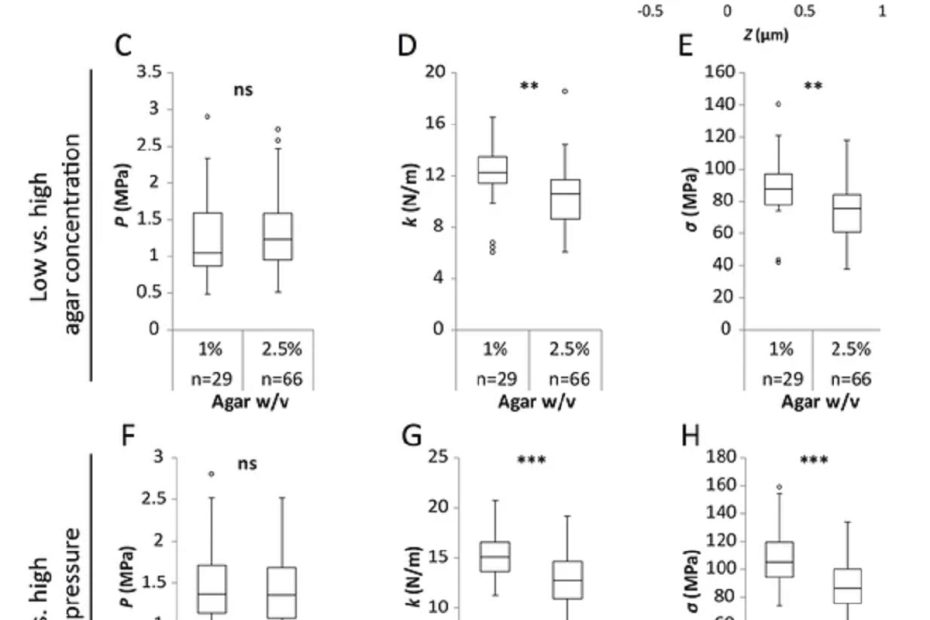
Elastic shell theory for plant cell wall stiffness reveals contributions of cell wall elasticity and turgor pressure in AFM measurement
The stiffness of a plant cell in response to an applied force is determined not only by the elasticity of the cell wall but also… Read More »Elastic shell theory for plant cell wall stiffness reveals contributions of cell wall elasticity and turgor pressure in AFM measurement