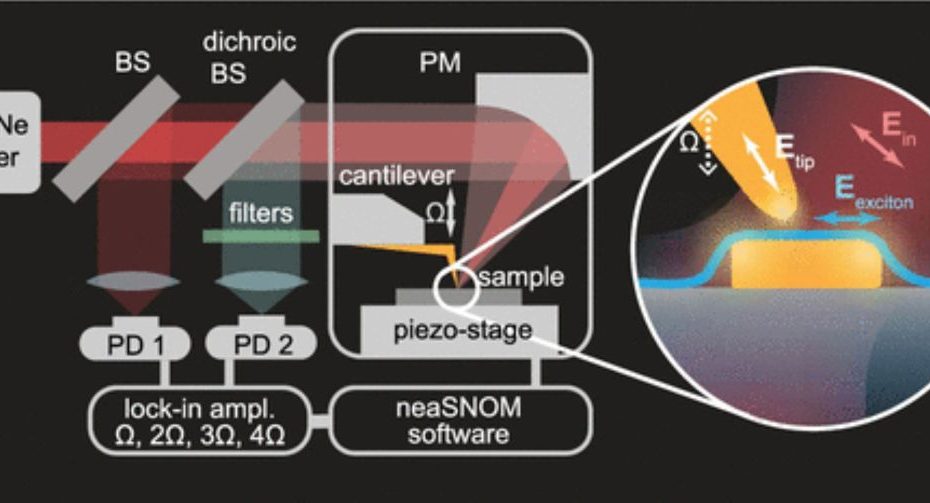
Enhanced Exiton-Plasmon Interaction Enabling Observation of Near-Field Photoluminescence in a WSe2-Gold Nanoparticle Hybrid System
Monolayer transition metal dichalcogenides, such as tungsten diselenide, have recently attracted considerable attention due to their reduced dielectric screening and direct bandgap, which result in… Read More »Enhanced Exiton-Plasmon Interaction Enabling Observation of Near-Field Photoluminescence in a WSe2-Gold Nanoparticle Hybrid System
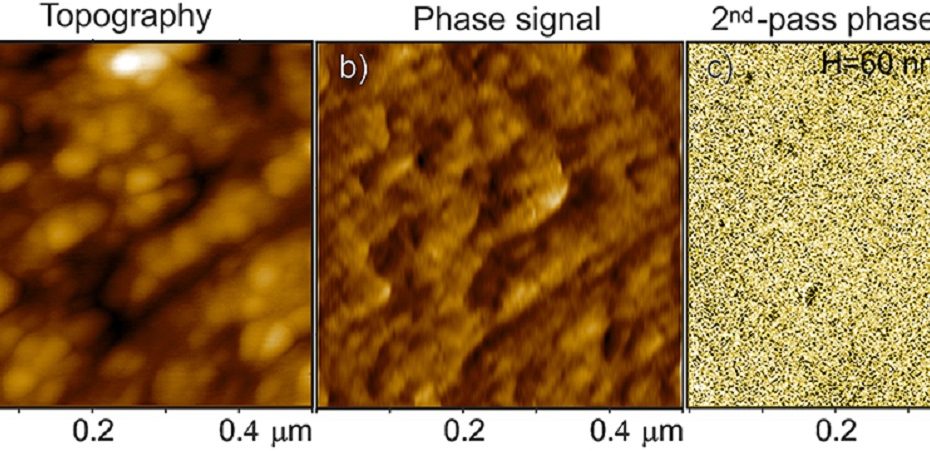
![Figure 5 from Catharina Husteden et al. 2023 “Lipoplex-Functionalized Thin-Film Surface Coating Based on Extracellular Matrix Components as Local Gene Delivery System to Control Osteogenic Stem Cell Differentiation”: A–D) Topography images [Cs/Col]4Cs (A), [Cs/Col]4Cs/LPX (B), [Cs/Col]4Cs/LPX/Cs (C), and [Cs/Col]4Cs/LPX/Cs/Col determined by AFM [Scale bar 1 µm] (D). E) Distribution curves of Young's modulus (E0) with a force map of a defined area (see also Figure S4, Supporting Information of the cited article). NANOSENSORSTM uniqprobe triangular qp-BioT AFM probes were used for the quantitative imaging to investigate the surface roughness and topography (in a standard liquid cell of a commercially available atomic force microscope) and force mapping. The uniqprobe qp-BioT AFM probe types offer an alternative to silicon nitride probes, with the advantage of taller AFM tips with smaller opening angles and reduced thermal drift.](https://d218f3btfcac6d.cloudfront.net/wp-content/uploads/2024/05/16205027/Fig-5-C-Husteden-et-al-2023-Lipoplex-Functionalized-Thin-Film-Surface-Coating-Based-on-Extracellular-Matrix-Components-NANOSENSORS-qp-BioT-AFM-probe-blog-930x620.jpg)

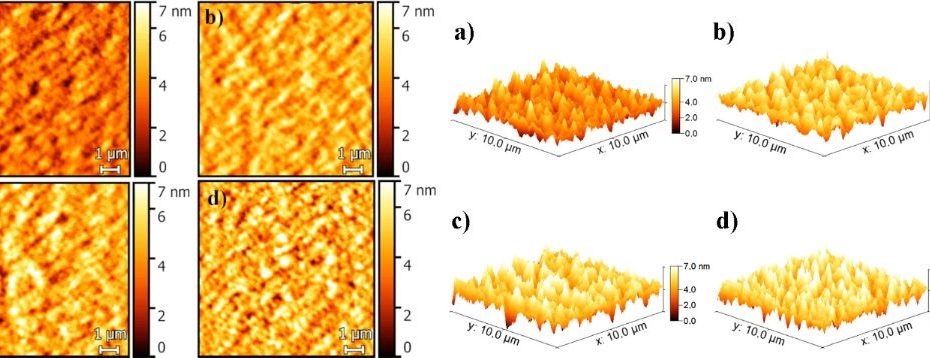

![Figure 5 from Yordanova et. al. "Zn2+-triggered self-assembly of Gonadorelin [6-D-Phe] to produce nanostructures and fibrils" - AFM image of the Zn2+: GnRH [6-D-Phe] 10:1 complex. (a,b) Oligomers after preparation with tapping mode in Tris buffer solution (c,d) fibrils with tapping mode in air (z-scale indicates the average size of the formed oligomers and fibrils). NANOSENSORS qp-BioAC AFM probe was used to perform images in buffer solution](https://d218f3btfcac6d.cloudfront.net/wp-content/uploads/2022/11/28103627/Figure_5_from_Zn2_triggered_self_assembly_of_Gonadorelin_to_produce_nanostructure_and_fibrils-2-900x620.png)