Nonlinear Biomechanical Characteristics of Deep Deformation of Native RBC Membranes in Normal State and under Modifier Action
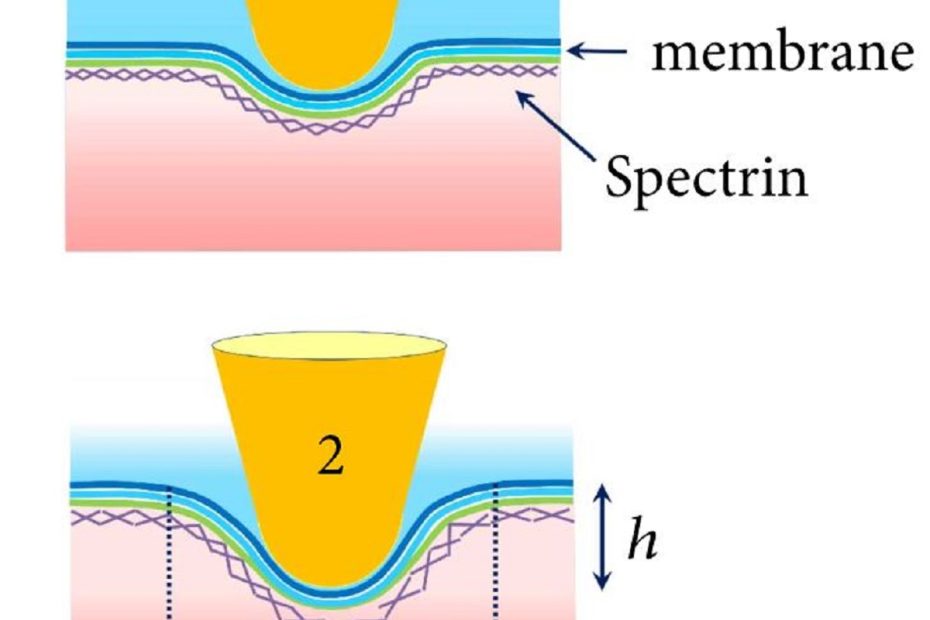
The mechanical properties and structural organization of membranes determine the functional state of red blood cells (RBCs). Deformability is one of the key physiological and… Read More »Nonlinear Biomechanical Characteristics of Deep Deformation of Native RBC Membranes in Normal State and under Modifier Action