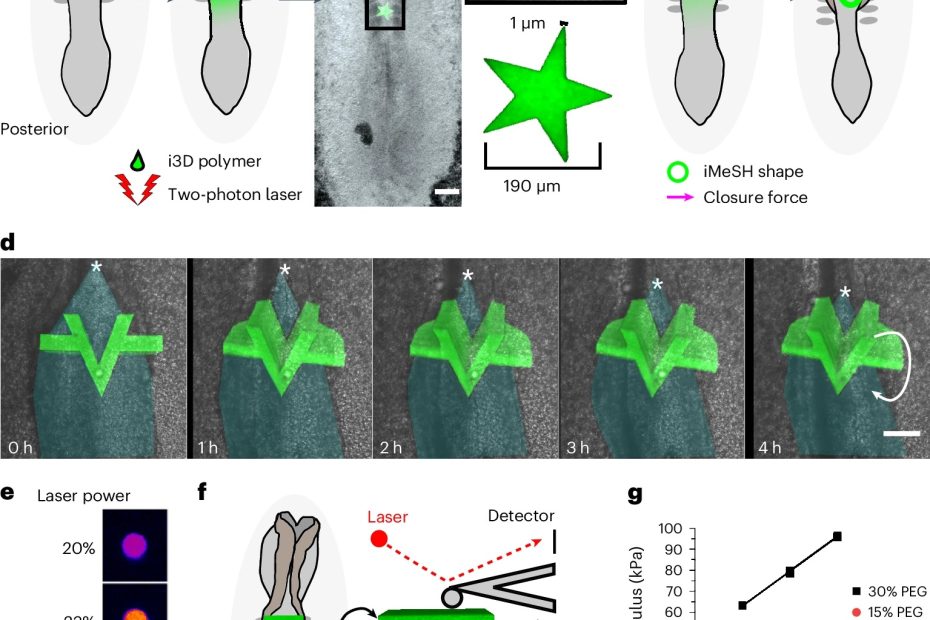
Quantifying mechanical forces during vertebrate morphogenesis
Morphogenesis requires embryonic cells to generate forces and perform mechanical work to shape their tissues. Incorrect functioning of these force fields can lead to congenital… Read More »Quantifying mechanical forces during vertebrate morphogenesis