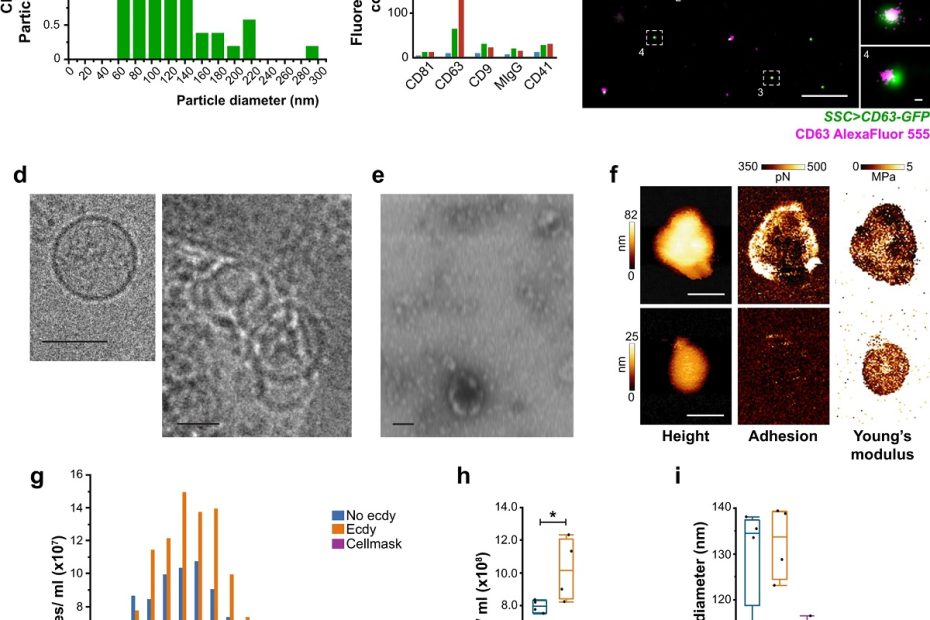
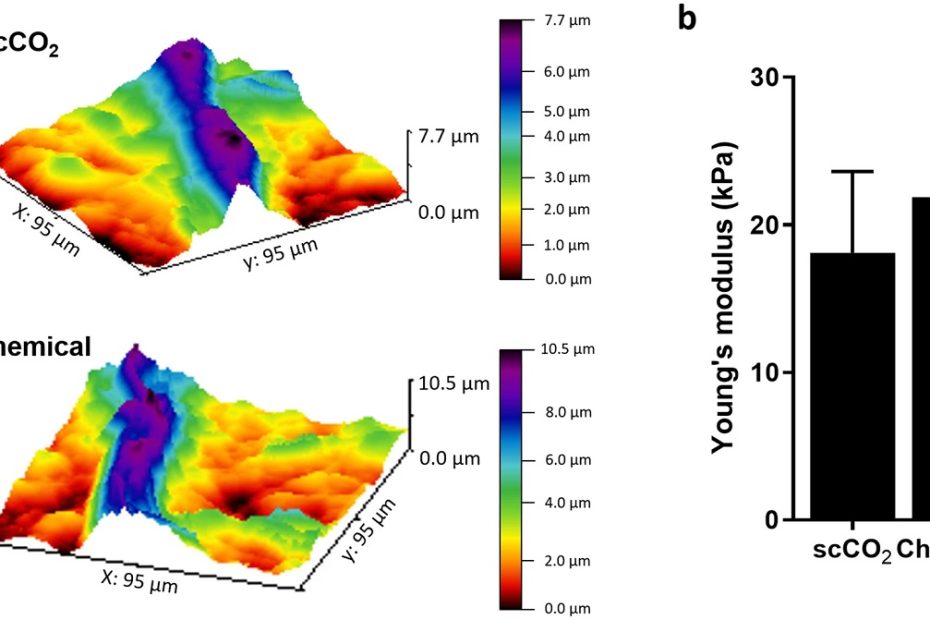
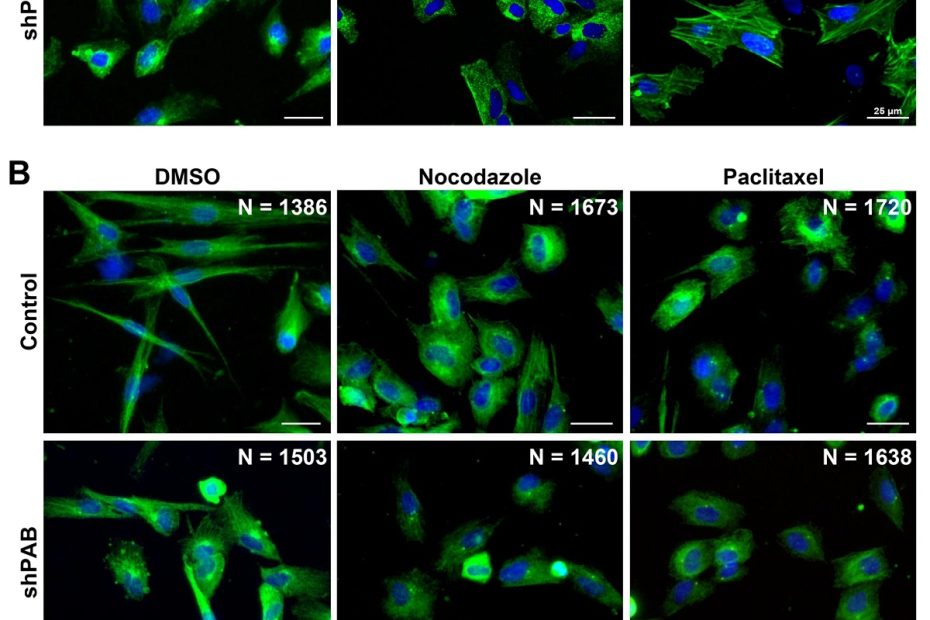
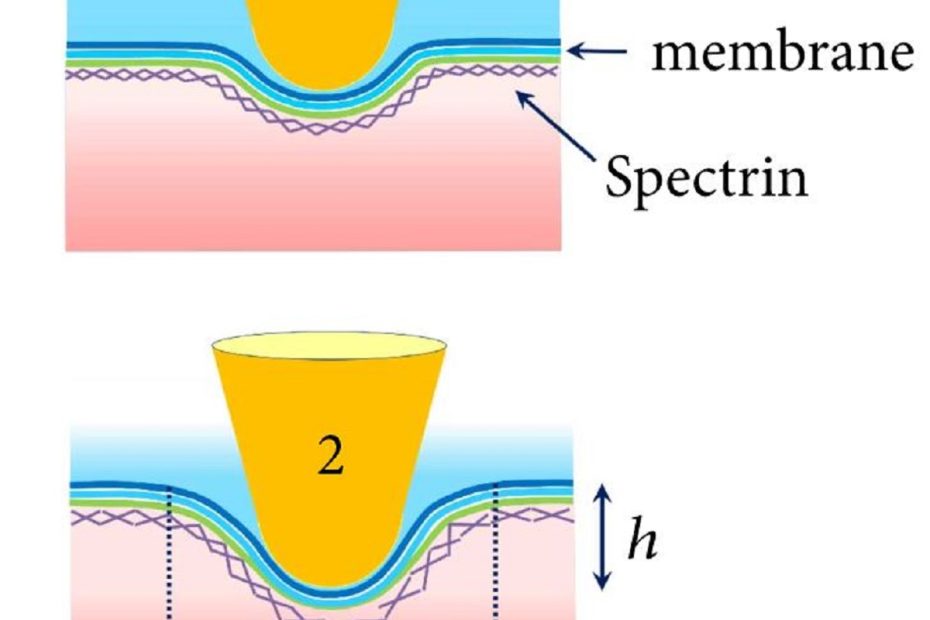
Male-female communication enhances release of extracellular vesicles leading to high fertility in Drosophila
The female reproductive tract (female-RT) must decipher the repertoire of molecular cues received from the male during copulation in order to activate and coordinate tract… Read More »Male-female communication enhances release of extracellular vesicles leading to high fertility in Drosophila