Bin Xue et al. have presented a novel class of biomimetic hydrogels that simultaneously exhibit high stiffness, toughness, fatigue resistance, and ultrafast mechanical recovery—properties that are notoriously difficult to combine in conventional polymeric systems. Their design is inspired by the architecture of mussel foot proteins and is centered around self-assembling peptide fibres termed “picot fibres”, which act as hierarchical load-bearing domains.
The hydrogel matrix consists of acrylamide-based polymer networks incorporating a custom-designed peptide (GK₁₁), which self-assembles into nanofibrous structures through β-sheet stacking. Upon introduction of Cu²⁺ ions, the histidine residues in the peptides undergo reversible metal coordination, forming sacrificial crosslinks that enhance mechanical robustness and energy dissipation.
These hydrogels (p-Pep/Cu²⁺) show a hidden-length release mechanism: under applied stress, the coordinated fibres elongate and unfold without permanent damage, then reassemble rapidly once the stress is removed. This molecular mechanism enables energy dissipation, stiffness retention, and self-repair on the timescale of milliseconds.
Comprehensive mechanical testing was performed, including uniaxial tensile and compressive loading, cyclic fatigue tests (over 10,000 cycles), crack propagation studies, and recovery rate measurements. Notably, the hydrogels demonstrate:
• Young’s modulus of ~71.5 kPa
• Fracture toughness up to ~38 MJ/m³
• Fatigue threshold ~424 J/m²
• >90% mechanical retention after 10⁴ cycles
• Recovery times in the sub-second range
Fig. 2: Characterisation of supramolecular and picot GK11 peptide fibres without and with metal Cu2+ coordination.
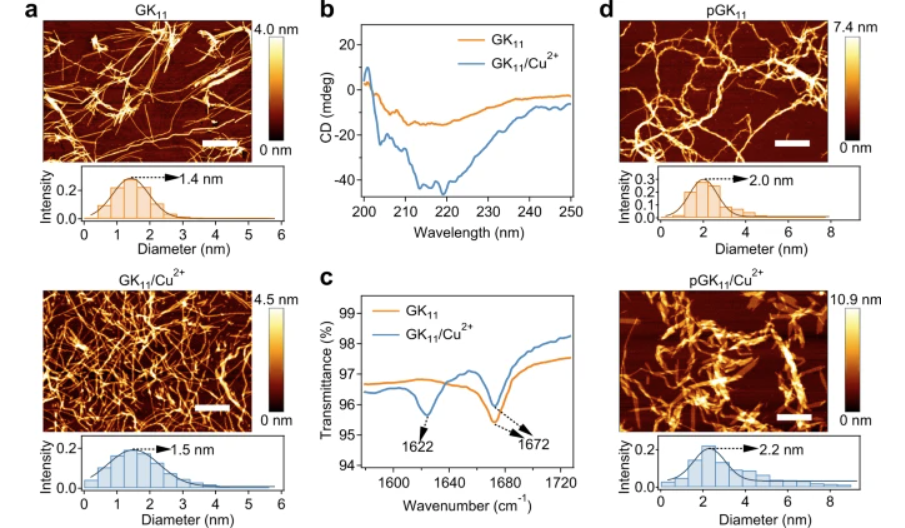
a AFM images of the fibres formed by the self-assembly of GK11 in the absence (top) and presence (bottom) of Cu2+. The graphs below the images correspond to the diameter distributions of the fibres. Scale bar = 1 μm. b, c CD spectrum (b) and FT-IR spectroscopy (c) of the self-assembled GK11 peptide with (GK11/Cu2+) and without Cu2+ (GK11). d As for (a), but for picot fibres (top) and metal ion-clad picot fibres (bottom) formed by GK11 linked with polyacrylamide in the absence and presence of Cu2+. Scale bar = 1 μm.
licensed under CC BY 4.0 Deed – Attribution 4.0 International
– Creative Commons
To probe the nanoscale mechanical properties and validate the role of fibre networks, the authors employed atomic force microscopy (AFM) in Quantitative Imaging (QI) mode using PPP-SEIHR silica cantilevers supplied by Nanosensors. These cantilevers, with a nominal spring constant of 42 N/m and tip radius ~10 nm, enabled high-resolution force–distance spectroscopy under well-controlled loading conditions..
The data revealed spatially heterogeneous stiffness distributions that correlated with fibre-rich domains. Mechanical mapping confirmed that Cu²⁺-coordinated fibre domains had significantly higher modulus and resilience compared to the amorphous matrix.
This work represents a breakthrough in hydrogel design by integrating reversible supramolecular interactions, peptide-based nanofibres, and metal ion coordination to overcome the stiffness–toughness trade-off. The use of Nanosensors PPP-SEIHR AFM probes was critical in verifying the presence and mechanical function of the peptide networks at the nanoscale, offering direct evidence for the proposed hidden-length mechanism.
Such materials have potential applications in soft robotics, wearable electronics, and biomedical implants, where mechanical durability, resilience, and responsiveness are paramount.
________________________________________
Citation
Xue, B., Bashir, Z., Guo, Y., et al. (2023). Strong, tough, rapid-recovery, and fatigue-resistant hydrogels made of picot peptide fibres. Nature Communications, 14, 2583.
🔗 https://www.nature.com/articles/s41467-023-38280-4