Surface chirality plays an important role in determining the biological effect.
Understanding how surface chirality regulates the behaviors of biological entities such as biomolecules and cells at the solid–liquid interface beyond the stereoselectivity is important but still not fully achieved.
In the article “Revealing the regulation of water dipole potential to aggregation of amyloid-β 42 at chiral interface by surface-enhanced infrared absorption spectroscopy” Manyu Zhu, Shanshan Li, Qixin Liu, Yuqi Zhang, Zihao Li, Yiran Wang, Lie Wu and Xiue Jiang investigate the different orientations of chiral molecules on gold nanofilm and reveal the effect of water at the chiral interface by combining surface-enhanced infrared absorption spectroscopy (SEIRAS), electrochemistry, and simulations. *
Their results reveal that water interacts more strongly with L-NIBC interface, and the adsorbed water shows positive dipole contribution, whose synergy results in a large shielding effect to the local negative potential, greatly weakening the electrostatic interaction between Aβ42 and L-NIBC interface and stimulating the hydrophobic interaction, which consequently induced the fibril aggregation. *
Conversely, the weaker compensation of water to the local potential of D-NIBC interface leads to the electrostatic adsorption of Aβ42, and the resultant spherical aggregates. *
The results presented by Manyu Zhu et al. not only point out for the first time the molecular nature of the chiral interface-regulated biological effect, including the different orientations of the chiral interface and the effect of water at the chiral interface but also demonstrate the dominant contribution of hydrophobic interaction to the fibril formation.
To better understand how the water at the chiral interface modulates the interaction between Aβ42 and NIBC interface and the subsequent aggregation, Manyu Zhu et al. tracked the fibrillation of Aβ42 on L(D)-NIBC surfaces using atomic force microscopy (AFM) after incubation in Aβ42 monomer solution (1 µm) for a different time at 37°C (Figure 3 cited in this blogpost). *
For AFM measurements, the L(D)-NIBC-modified mica were incubated with 1 µm Aβ42 for different times and then were thoroughly washed with 10 mm PBS and ultra-pure water, respectively. After that, the substrates were dried with high-purity nitrogen for measuring the morphology of Aβ42 by AFM in tapping mode with NANOSENSORS PointProbe® Plus PPP-NCHR AFM probes.*
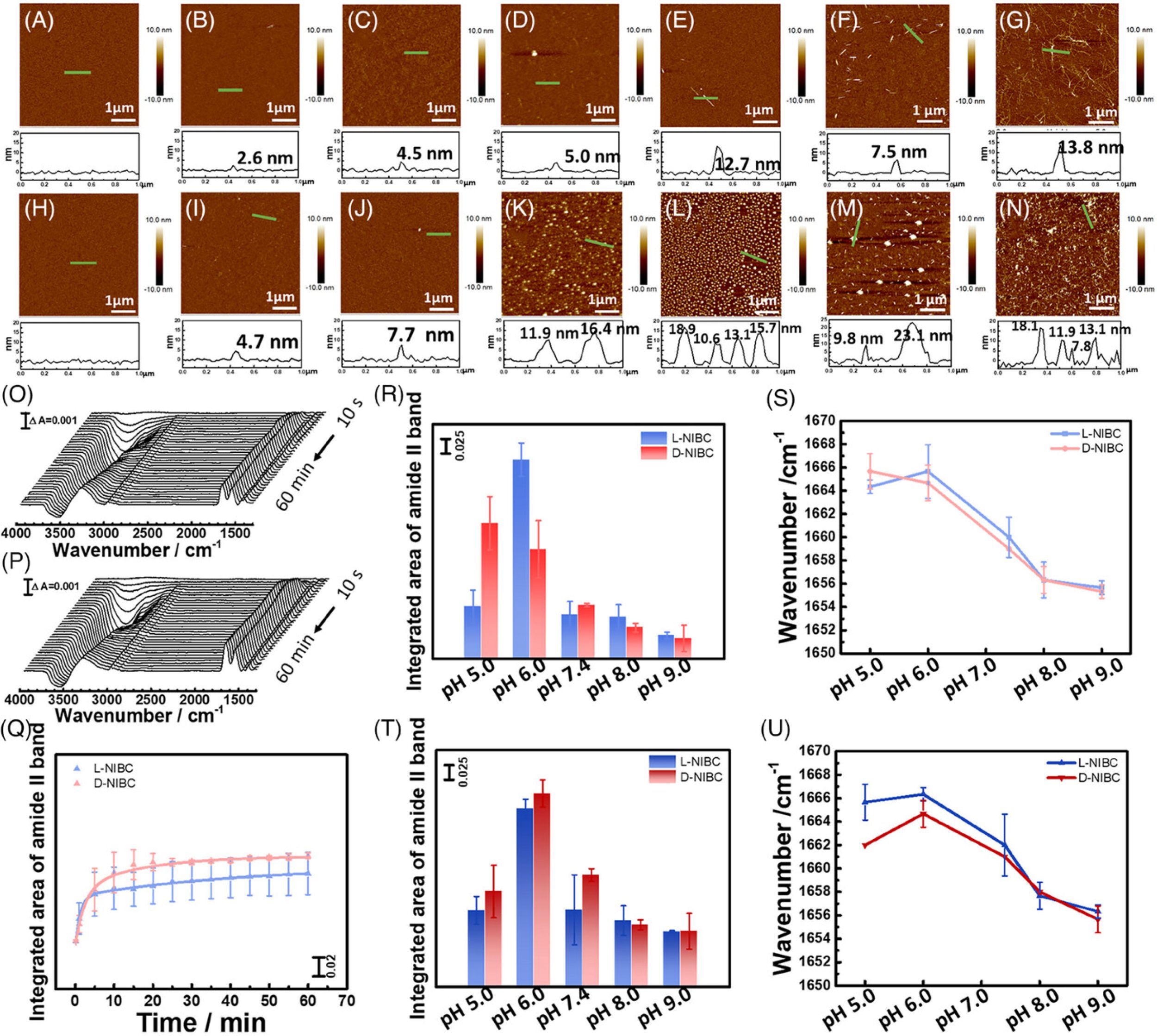
Figure 3 from Manyu Zhu et al. (2024), “Revealing the regulation of water dipole potential to aggregation of amyloid-β 42 at chiral interface by surface-enhanced infrared absorption spectroscopy”
Aggregation of Aβ42 on different chiral interfaces. (A–E) AFM images of L-NIBC surface and (H–L) D-NIBC surface after incubation in Aβ42 monomer solution for 0 min, 10 min, 1 h, 12 h, 24 h at 37°C in the presence of 10 mm PBS at pH 7.4. (F,M) AFM images of L-NIBC surface and D-NIBC surface after incubation in Aβ42 monomer solution in the presence of 10 mm PBS at pH 9.0. (G,N) AFM images of L-NIBC surface and D-NIBC surface after incubation in Aβ42 monomer solution in the presence of 10 mm PBS and 150 mm NaCl at pH 7.4. (O,P) Time-dependent SEIRA spectra of Aβ42 on L-NIBC interface and on D-NIBC interface. (Q) Time-dependent surface coverage of Aβ42 on L (blue)/D-NIBC (red) chiral interfaces in the presence of 10 mm PBS at pH 7.4, which was calculated by integrating the area of the amide II band. (R) Integrated area of amide II band on L (blue)/D-NIBC (red) chiral interfaces in the presence of 10 mm PBS at pH 5.0, 6.0, 7.4, 8.0, 9.0, respectively. (S) Amide I band peak position on L (blue)/D-NIBC (red) chiral interfaces in the presence of 10 mm PBS at pH 5.0, 6.0, 7.4, 8.0, 9.0, respectively. (T) Integrated area of amide II band on L (blue)/D-NIBC (red) chiral interfaces in the presence of 10 mm PBS and 150 mm NaCl at pH 5.0, 6.0, 7.4, 8.0, 9.0, respectively. (U) Amide I band peak position on L (blue)/D-NIBC (red) chiral interfaces in the presence of 10 mm PBS and 150 mm NaCl at pH 5.0, 6.0, 7.4, 8.0, 9.0, respectively.
*Manyu Zhu, Shanshan Li, Qixin Liu, Yuqi Zhang, Zihao Li, Yiran Wang, Lie Wu and Xiue Jiang
Revealing the regulation of water dipole potential to aggregation of amyloid-β 42 at chiral interface by surface-enhanced infrared absorption spectroscopy
Aggregate, Volume 5, Issue 5, October 2024, e601
DOI: https://doi.org/10.1002/agt2.601
The article “Revealing the regulation of water dipole potential to aggregation of amyloid-β 42 at chiral interface by surface-enhanced infrared absorption spectroscopy” by Manyu Zhu, Shanshan Li, Qixin Liu, Yuqi Zhang, Zihao Li, Yiran Wang, Lie Wu and Xiue Jiang is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third-party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/.